Findings
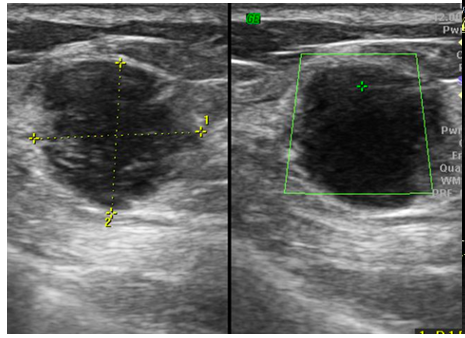
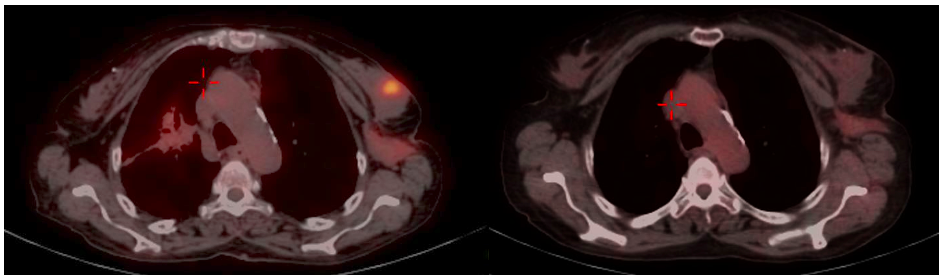
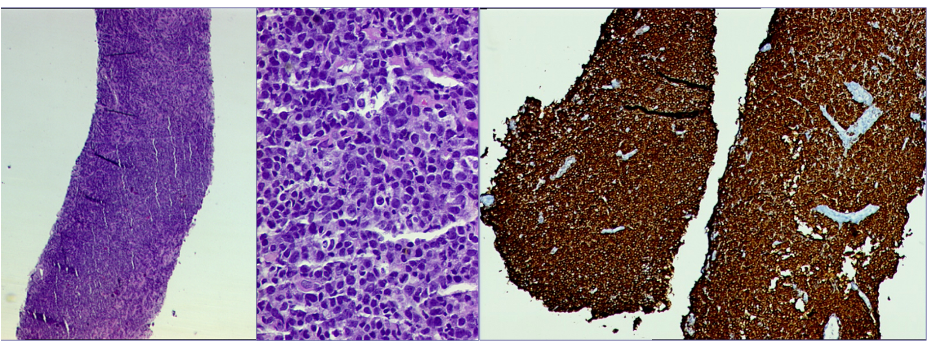
Mammography (Figure 1)revealed a large, round, well circumscribed, high density mass in upper outer quadrant and central region, measuring 7 x 6 cm. No suspicious calcification, architectural distortion, nipple retraction, skin or nipple-areolar thickening was seen. Associated was a large node measuring 11 x 9 cm noted in the left axillary region. Right breast and axilla were unremarkable. Correlation USG images were not available on Electronic Medical Record and PACS system, hence not available for reproduction here, however, as per the report, it appeared as a lobulated hypoechoic mass in left breast with necrotic changes, and internal vascularity in the solid component. Similar morphology additional left axillary mass was also seen. Imaging features highlighted the absence of spiculation, calcification, architectural distortion, irregular margins. The mass was categorized as BIRADS 4c, with a high probability of malignancy.Differentials on basis of mammography and USG were triple negative breast carcinoma, phyllodes tumor, cellular fibroepithelial lesion, organized abscess, lymphoma or metastases. CT was done to see disease extent which revealed a heterogeneously enhancing left breast mass (Fig 2a), large axillary and retropectoral adenopathy. No other adenopathy or abnormality seen. Biopsy from the breast lump was performed which showed Non-Hodgkin’s lymphoma of diffuse large B cell type, positive for CD20 and Mum1 which are markers of mature B cells. CSF study and Bone marrow aspiration were negative for involvement by disease. Diagnosis of primary breast lymphoma was made and planned for chemotherapy R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine and prednisone) followed by IFRT (Involved field radiotherapy). Post 3 cycles of chemotherapy, FDG PET (Fig 2b and 3a) was done which showed significant reduction in size of breast mass and axillary node, breast mass then measuring 2 x 2cm with SUVmax of 7 while the axillary node measured 3.4 x 2.6 cm with SUV max of 3.7. Another repeat FDG PET Scan (Fig 2c and 3b)was done after completion of chemotherapy for further response assessment which showed no FDG uptake in left breast lesion suggesting complete metabolic response however size of the lesion appeared nearly stable, as compared to previous FDG PET (performed in the interim period of therapy). Patient has a 2-year disease free interval, and has not visited due to the Covid pandemic situation, but on telephonic follow up, is asymptomatic with good performance status. Figure 4: Low power (a), High power (b) and CD20 staining (c) from another case of primary breast lymphoma. These are representative images added here for teaching purpose. Figure 2a (10x power) shows diffuse infiltration by malignant cells. Figure 2b shows monomorphic round cells with apoptosis. Figure 2c staining positive for phenotype analysis for B cells. Representative USG image (Figure 5 and 6) of different patients with biopsy proven B cell lymphoma with different demographics. (Figure 5) USG of breast lump in a 56 year old woman shows hypoechoic, round mass, circumscribed margin with posterior acoustic enhancement mimicking a receptor negative carcinoma, mucinous carcinoma or fibroepithelial lesion. (Fig 6) Large mass in a 21 years old lady shows mixed echogenicity oval mass of parallel orientation, with well circumscribed margin and surrounding echogenic rim due to edema, mimicking a phylloides tumour, a fibroepithelial lesion or a malignant etiology.
Answer
Differential diagnoses: Triple negative breast carcinoma, Phyllodes tumor, Cellular fibroepithelial lesion, Organized abscess, Lymphoma or Metastases. Diagnosis: Primary Breast Lymphoma
Discussion
Breast lymphoma is a rare entity accounting for approximately 0.04% to 0.7% of all breast cancer cases1. Breast lymphoma may occur as either a primary or a secondary lesion. Primary breast lymphoma is diagnosed when breast without or with ipsilateral axilla are the only site of disease and no evidence of lymphoma elsewhere. Median age of diagnosis of the primary breast lymphoma is 60-65 years2. The most common symptom of breast lymphoma is a painless, palpable mass. Ipsilateral axillary lymphadenopathy may be associated. Breast lymphoma on mammography appears as a solitary, round or oval, noncalcified hyper or isodense mass with circumscribed or indistinct margins. Adjacent asymmetry or architectural distortion are less frequent. It may also appear as diffuse increased breast density, multiple masses or a smaller mass2,3. Ultrasound features of breast lymphoma are non-specific and include hypoechoic, oval or round mass with circumscribed or indistinct margin in parallel orientation. Mixed echogenicity, posterior acoustic enhancement, echogenic rim due to lymphedema can be seen in some cases. These often show increased vascularity on doppler USG1,2. FDG PET/CT shows variable FDG uptake in lymphoma, and is essentially used for disease staging and response assessment. It is reportedly superior in detecting lesions in lymphoma as compared to Ga 67 SPECT4. Semiquantitative evaluation of lymphoma can be done by SUV value for response assessment. Higher SUV value (more than 10) is indicative of aggressive lymphoma whereas low grade lymphoma can have low SUV (less than 6)5. No Metabolic activity in lymphoma is considered as complete metabolic response whereas; a more than 50% fall in SUV value from pre-treatment value suggests significant response6. Surveillance of primary breast lymphoma, after complete metabolic response at our institute is based on clinical examination, every 3 to 4 months for the first year, followed by 6 monthly assessment for the next. Surveillance mammogram is also suggested. Treatment for B-cell lymphoma is a multi-agent regimen consisting of anthracycline-based chemotherapy with rituximab; combined with radiation. Lumpectomy or mastectomy offers no added benefit from in treatment of breast lymphoma2. Primary breast lymphoma has better overall survival than secondary breast lymphoma and carcinoma breast. Surveillance of lymphoma is based on factors such as grade of lymphoma, risk of radiation exposure, patient compliance and cost of surveillance. 7 Differential diagnosis Various lesions can mimic imaging features of lymphoma on mammography including triple negative breast carcinoma (TNBC), metastases, giant fibroadenoma, phyllodes tumor, organised abscess. TNBC are commoner in middle-age women, presenting as a well circumscribed mass with absence of calcifications on mammography and hypoechoic lesion with variable posterior acoustic shadowing or enhancement on USG. Like benign lesions, TNBC also may lack suspicious features like spiculation, microcalcifiaction and architectural distortion. These often mimic benign lesions when they are of smaller size. Cellular fibroepithelial lesions occur in younger patients as compared to lymphoma. Clinical and imaging features are similar for both these lesions and HPR correlation is gold standard. Phyllodes tumors are prevalent in younger patients than lymphoma, both have similar mammographic appearance. USG features like lobulations with fluid clefts and posterior acoustic enhancement can help predict a diagnosis of phyllodes. Organised abscess without moving debris is another differential of lymphoma which can occur at any age. Metastases to breast can resemble lymphoma and common sites of metastases to breast are systemic lymphoma (most common), melanoma, sarcoma, lung, gastric, ovarian and renal cancers. Systemic staging is essential for detection of primary lesion before diagnosis of primary breast lymphoma. While assessing the possible differentials, the age group of the patient can help in narrowing differentials on imaging. FDG PET is reserved for response evaluation and not used for diagnosis of breast lesion. High FDG activity similar to lymphoma can be seen in larger masses, high-grade tumors and in TNBC. Hence, histopathological evaluation helps reach the diagnosis. Conclusion Lymphoma of breast is a rare entity with nonspecific imaging features. Large solitary well circumscribed mass in old age women should raise its possibility with biopsy correlation to avoid unnecessary upfront surgical intervention. It is imperative to understand that the role of FDG PET is not just for assessment for aggressive nature, but is a surrogate marker for assessing complete response, despite residual fibrotic or necrotic mass on CT scan, which must not be mistaken as poor response.
Reference
1. Surov, A. et al. Primary and secondary breast lymphoma: Prevalence, clinical signs and radiological features. Br. J. Radiol. (2012) doi:10.1259/bjr/78413721. 2. Raj, S. D. et al. Primary and secondary breast lymphoma: Clinical, pathologic, and multimodality imaging review. Radiographics (2019) doi:10.1148/rg.2019180097. 3. Shim, E., Song, S. E., Seo, B. K., Kim, Y. S. & Son, G. S. Lymphoma affecting the breast: A pictorial review of multimodal imaging findings. Journal of Breast Cancer (2013) doi:10.4048/jbc.2013.16.3.254. 4. Kostakoglu, L., Agress, H. & Goldsmith, S. J. Clinical Role of FDG PET in Evaluation of Cancer Patients. Radiographics (2003) doi:10.1148/rg.232025705. 5. D’Souza, M. D. et al. FDG-PET/CT in lymphoma. Indian J. Radiol. Imaging (2013) doi:10.4103/0971-3026.125626. 6. Santra, A. et al. FDG PET-CT in the management of primary breast lymphoma. Clin. Nucl. Med. (2009) doi:10.1097/RLU.0b013e3181becdfc. 7. Kallam, A., Adusumalli, J. & Armitage, J. O. Surveillance in Patients With Diffuse Large B Cell Lymphoma. Mayo Clinic Proceedings (2020) doi:10.1016/j.mayocp.2019.05.011.